OVERVIEW
Glioblastoma (GBM), also referred to as a grade IV astrocytoma, is a fast-growing and aggressive brain tumor. It invades the nearby brain tissue, but generally does not spread to distant organs.
GBMs can arise in the brain de novo or evolve from lower-grade astrocytoma. In adults, GBM occurs most often in the cerebral hemispheres, especially in the frontal and temporal lobes of the brain. GBM is a devastating brain cancer that can result in death in six months or less, if untreated; hence, it is imperative to seek expert neuro-oncological and neurosurgical care immediately, as this can impact overall survival.
GBMs present unique treatment challenges due to:
- Localization of tumors in the brain
- Inherent resistance to conventional therapy
- Limited capacity of the brain to repair itself
- Migration of malignant cells into adjacent brain tissue
- The variably disrupted tumor blood supply, which inhibits effective drug delivery
- Tumor capillary leakage, resulting in an accumulation of fluid around the tumor, (peritumoral edema) and intracranial hypertension
- Tumor-induced seizures
- The resultant neurotoxicity of treatments directed at gliomas
Prevalence and Incidence
Glioblastoma is the most common malignant brain and other CNS tumors accounting for 47.7% of all cases. Glioblastoma has an incidence of 3.21 per 100,000 population.
Median age of diagnosis is 64 years and it is more common in men as compared to women. Survival is poor with approximately 40% survival in the first year post diagnosis and 17% in the second year.
Factors associated with glioblastoma risk are prior therapeutic radiation, decreased susceptibility to allergy and impaired immune response. Several hereditary cancer syndromes greatly increase the risk of glioblastoma, including Li-fraumeni syndrome and Lynch syndrome.
Symptoms
Symptoms vary depending on the location of the brain tumor, but may include any of the following:
- Persistent headaches
- Double or blurred vision
- Vomiting
- Loss of appetite
- Changes in mood and personality
- Changes in ability to think and learn
- New onset of seizures
- Speech difficulty of gradual onset
Diagnosis
Sophisticated imaging techniques can accurately pinpoint the location of brain tumors. Diagnostic tools include computed tomography (CT or CAT scan) and magnetic resonance imaging (MRI). Intraoperative MRI may also be useful during surgery to guide tissue biopsies and tumor removal. Magnetic resonance spectroscopy (MRS) is used to examine the tumor’s chemical profile.
Conventional MRI: Magnetic resonance imaging (MRI) is the most important imaging study for astrocytoma. Usually, images are acquired both before and after the administration of IV contrast. As a rule of thumb, if the tumor picks up the contrast (i.e. becomes bright on images) is an indication of a higher-grade astrocytoma.
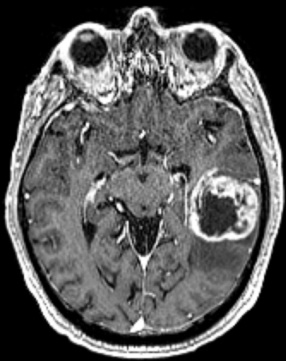
Other imaging sequences provide clues as to tumor cellularity, brain swelling and brain infiltration. Low-grade tumors usually do not show much contrast enhancement, while GBMs display strong contrast enhancement and frequent central necrosis (Figure 1).
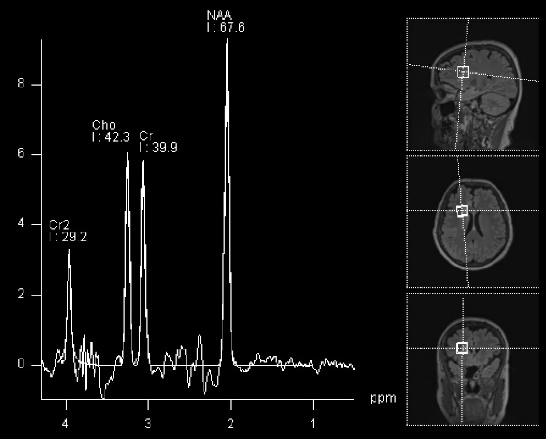
MRI spectroscopy (MRS): This is an imaging tool, based on MRI, that provides information on the chemical composition of the tumor and works based on the fact that certain chemicals are abundant in the normal brain, while others are abundant in tumors (for example, choline). The output of this imaging modality is a diagram where it is possible to see the amount of each chemical in an area of the brain under analysis: If the amount of NAA is more than choline, that would suggest a normal brain (Figure 2). The opposite raises suspicion of a tumor. This technique can be considered as a non-invasive tissue sampling, although it is not as accurate or definitive as a standard biopsy.
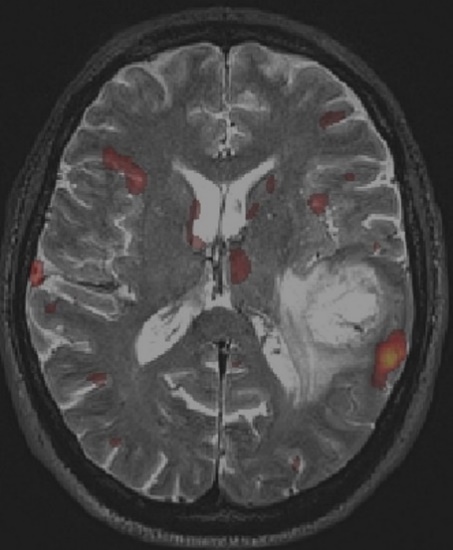
Functional MRI (fMRI): fMRI is a useful technique to find which parts of the brain become activated when the patient is asked to perform a certain task (for example, talking or moving one arm or leg). This is fundamental to define the regions of the brain which, if damaged, would cause problems to the patient. Activated brain is shown as a yellow/red signal (Figure 3) superimposed to an otherwise standard MRI. For tumors that are localized in the proximity of critical areas (speech centers, motor cortex or visual cortex), fMRIs provide an important adjunct, particularly in regards to surgical planning.
The yellow/red signal displays significant activation in the left temporo-parietal region, in the expected anatomical area for language production and in close proximity to the location of a glioblastoma.
Grading
After a brain tumor is detected on a CT or MRI scan, a neurosurgeon obtains tumor tissue for a biopsy and the tissue is examined by a neuropathologist.
The analysis of tumor tissue is used to assign the tumor a name, grade and to provide answers to the following questions:
- What is the type of tumor and how is it classified based on the WHO tumor classification?
- Are there signs of rapid growth in the tumor cells? What is the tumor grade?
| Histologic Grading | |
| GRADE II | Cytologic atypia (variation in nuclear shape and size + hyperchromasia) |
| GRADE III | Anaplasia and increased mitotic activity (increased cellularity) |
| GRADE IV | Microvascular proliferation and necrosis |
- Are there any specific genetic mutations within the tumor that can help with prognosis, help predict response to therapy and assess presence of experimental therapeutic targets?
Next generation sequencing aids molecular analysis and in profiling brain tumors to improve diagnostic accuracy, therapeutic target identification and predict prognosis. A few important alterations are in the table below.
| Important Molecular Alterations in Glioblastoma | |
| IDH mutation | Prognostic value, potential therapeutic target |
| MGMT methylation status | Prognostic value, predictive value for response to temozolomide |
| EGFR mutation | Diagnostic maker for glioblastoma, potential therapeutic target |
| TERT promoter mutation | Diagnostic maker for glioblastoma |
| Gain for 7p and loss of 10q | Diagnostic maker for glioblastoma |
| H3F3A | Diagnostic marker for a subset of gliomas (H3 K27M-mutant and H3 G34 mutant), therapeutic target |
| FGFR fusion | Therapeutic target |
| NTRK fusion | Therapeutic target |
Treatment Options
The mainstay of treatment for GBMs is surgery, followed by radiation and chemotherapy. The primary objective of surgery is to remove as much of the tumor as possible without injuring the surrounding normal brain tissue needed for normal neurological function. However, GBMs are surrounded by a zone of migrating, infiltrating tumor cells that invade surrounding tissues, making it impossible to ever remove the tumor entirely. Surgery provides the ability to reduce the amount of solid tumor tissue within the brain, remove those cells in the center of the tumor that may be resistant to radiation and/or chemotherapy and reduce intracranial pressure. Surgery, by providing a debulking of the tumor, carries the ability to prolong the lives of some patients and improve the quality of remaining life.
In most cases, surgeons perform a craniotomy, opening the skull to reach the tumor site. This is done frequently with computer-assisted image-guidance and at times using intra-operative mapping techniques to determine the locations of the motor, sensory and speech/language cortex. Intraoperative mapping often involves operating on a patient while they are awake and mapping the anatomy of their language function during the operation. The doctor then decides which portions of the tumor are safe to resect.
After surgery, when the wound is healed, radiation therapy can begin. The goal of radiation therapy is to selectively kill the remaining tumor cells that have infiltrated the surrounding normal brain tissue. In standard external beam radiation therapy, multiple sessions of standard-dose “fractions” of radiation are delivered to the tumor site as well as a margin in order to treat the zone of infiltrating tumor cells. Each treatment induces damage to both healthy and normal tissue.
By the time the next treatment is given, most of the normal cells have repaired the damage, but the tumor tissue has not. This process is repeated for a total of 10 to 30 treatments, usually given once a day, five days a week; depending on the type of tumor. The use of radiation therapy provides most patients with improved outcomes and longer survival rates compared to surgery alone or the best supportive care.
Radiosurgery is a treatment method that uses specialized radiation delivery systems to focus radiation at the site of the tumor, while minimizing the radiation dose to the surrounding brain. Radiosurgery may be used in select cases for tumor recurrence, often using additional information derived from MRS or PET scans. It is rarely used in the initial treatment of GBM.
Patients undergoing chemotherapy are administered special drugs designed to kill tumor cells. Chemotherapy with the drug temozolomide is the current standard of treatment for GBM. The drug is generally administered every day during radiation therapy and then for six cycles after radiation during the maintenance phase. Each cycle lasts for 28 days, with temozolomide given the first five days of each cycle, followed by 23 days of rest. Tumor treating fields is a different modality of treatment that is introduced during the maintenance phase of treatment. It creates alternating electrical fields, which prevents growth and division of cancer cells. Lomustine (chemotherapy) and bevacizumab (targeted therapy) are largely used when the tumor progresses.
Note from AANS
The AANS does not endorse any treatments, procedures, products or physicians referenced in these patient fact sheets. This information provided is an educational service and is not intended to serve as medical advice. Anyone seeking specific neurosurgical advice or assistance should consult his or her neurosurgeon, or locate one in your area through the AANS’ Find a Board-certified Neurosurgeon online tool.
